


SafetyTector® S is a SARS-CoV-2 inactivating extraction and dilution buffer for saliva specimens and nasopharyngeal swabs. Swabs can be extracted directly with SafetyTector® S. Samples must be diluted in SafetyTector® S at least 1:4. At this dilution, SafetyTector® S is able to inactivate SARS-CoV-2 within 1 minute. Therefore the samples are no longer infective.
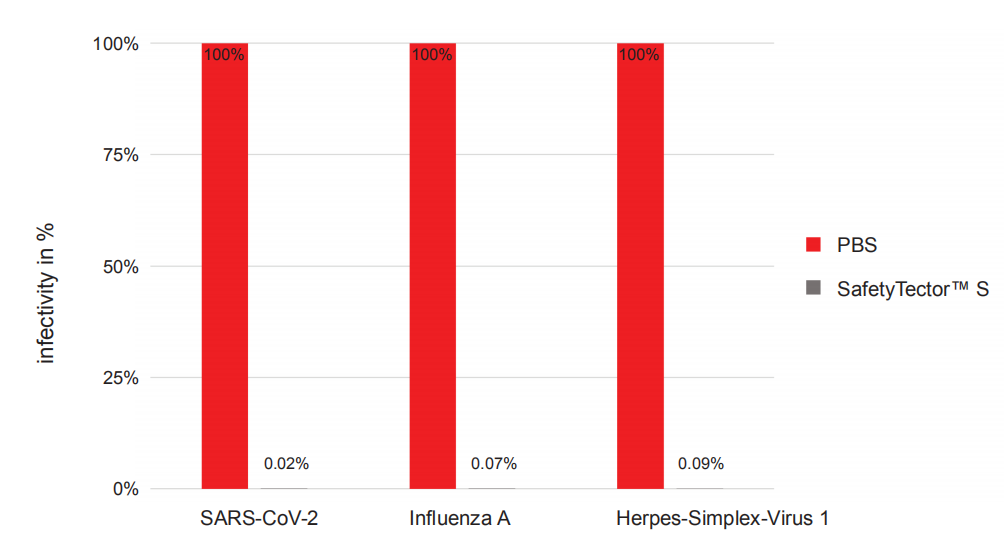
SafetyTector® S eliminates the high risk of infection during and after testing coming from the specimen or other test materials. Virus inactivation was confirmed and tested with infectious viruses in a Biosafety Level 3 laboratory.
SafetyTector® S completely eliminates infectivity of SARS-CoV-2
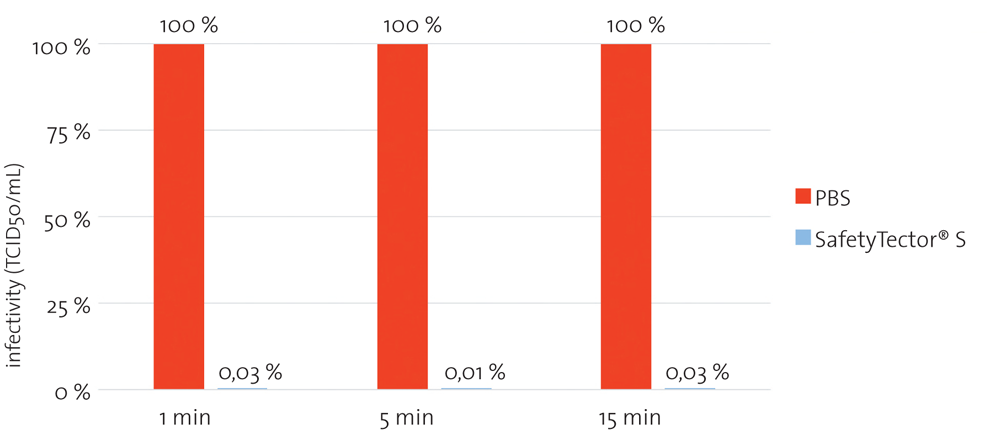
Saliva samples spiked with SARS-CoV-2 were mixed 1:4 with PBS or CANDOR‘s SafetyTector® S and incubated for the indicated times at room temperature. After incubation, samples were titrated and added on Vero E6 cells. After 5 to 7 days, infectivity was determined according to the Reed-Muench-method. Already after 1 min of incubation with SafetyTector® S, no remaining infectivity was detectable with this assay.
Samples diluted in SafetyTector® S can directly be used in lateral flow assays and also in other immunoassays such as ELISA, protein arrays or bead-based assays.
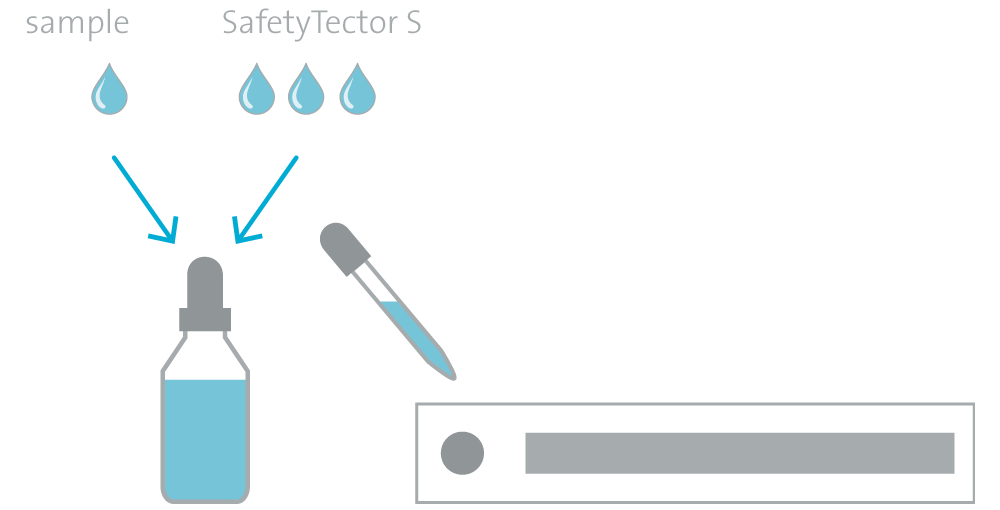
In tests with commercially available SARS-CoV-2 antigen rapid tests, SafetyTector® S shows comparable or better performance than supplied extraction and dilution buffers. In some cases, further improvement in the detection limit was observed.
The suitability of SafetyTector® S for the respective assay has to be tested by the manufacturer or distributor.
Safe Design of in vitro diagnostic medical devices (IVD products) is an obligatory pre-requisite for placing them on the international markets in the EU. This is defined in EU legislation (Directive 98/79/EG, Annex I, chapter B, clause 2.1 and Regulation (EU) 2017/746, Annex I, Chapter I, clause 4). Besides that, the ISO 14971 „application of risk management to medical devices“ (ISO 14971:2012, Annex A, chapter A.2.6 risk control) - decisive for the correct implementation of the ISO 13485 - mandates a risk control procedure and thus gives another regulatory binding basis for ensuring that IVDs are safe and secure for the user in all international markets. Safe Design includes that the risk of infection for the user is eliminated or reduced to the lowest possible level – this is especially important for IVDs for highly infectious viruses such as SARS-CoV-2. The current pandemic situation with the highly infectious variants of SARS-CoV-2 forces immediate action even for IVD products already on the market.
Virus-inactivating extraction buffers are an easy-to-use and effective tool to produce IVDs in conformity with these mandatory regulations.
During the past there were no virus-inactivating extraction buffers, proven to be able to inactivate SARS-CoV-2 by experiments under real conditions with infective viruses, available on the market. SafetyTector® S thus sets a current state of the art in terms of safe design for user safety during antibody- and antigen-tests – especially in rapid lateral flow testing. Testing virus inactivation with substitute test materials such as virus-like particles (VLPs) cannot safely allow the same conclusions in terms of user safety.
Together with SafetyTector® S manufacturers of IVD directly get the necessary evidence for virus inactivation in form of a “Letter of Acknowledgement” from the testing Biosafety Level 3 laboratory.
